
Let's look at a qualitative example explaining the electronic structure of the nitric oxide, NO, molecule. The MOs obtained by combining atomic orbitals that have a nodal plane on the bond axis, retain this same nodal plane and are called #pi#.Ħ) The progressive occupation of MOs follows the same rules as that of atomic orbitals. They are usually represented by adding an asterisk ( #"*"#) to the name of the orbital.ĥ) The MOs obtained by a linear combination of atomic orbitals that are symmetrical with respect to the bond axis (the orbitals #s# and one of the #p#) have the same symmetry and will be designated as orbitals #sigma#. The other half are called anti-bonding and have more energy than the atomic orbitals from which they come. We can imagine the LCAO as a linear transformation that changes the base, obtaining one in which the energy of the system is minimal.Ĥ) Half of the molecular orbitals obtained by combining atomic orbitals of the same or similar energy are more stabilized (have less energy) than these and we will call them bonding. Molecular orbitals will in turn be a new base of said subspace, constructed in such a way that the energy of the set is minimized. This means that we are going to combine linearly the #n# atomic orbitals available to obtain #n# molecular orbitals.įrom a mathematical point of view, we will say that the #n# atomic orbitals form a basis of a subspace of dimension #n# within Hilbert space. In formula we can see that both #i# and #j# vary between #1# and #n#. This implies that, in keeping with the remaining quantum numbers, we must at least have the spin quantum number different, as in atomic orbitals, and therefore we can only accommodate two electrons in each orbital.ģ) The linear combination of a given number of atomic orbitals gives the same number of molecular orbitals. Therefore, in the same orbital, two electrons with identical wave functions can not coexist. The Pauli exclusion principle is applicable to molecular orbitals since any wave function describing the motion of an electron must meet the property of being antisymmetric since electrons are fermions. It would be the case of the hydrogen atom and the hydrogen like atoms (atoms with #Z# protons in the nucleus and a single electron, like #He^+#, #Li^ #Ģ) A molecular orbital is complete if it contains two electrons. In the specific case of the study of the atomic and molecular structure, where the electrostatic interactions predominate, the Schrödinger equation for a monoelectronic atom has an exact solution. In this context is known the old problem of the movement of three bodies subjected to gravitational interactions between them, which was soon found to have no exact mathematical solution. Since Newton's time we know that when we have a system with more than two particles interacting with each other by central forces, there is no exact analytical solution of the equations of the motion of these particles. A molecule, in its ground state, will always first fill the bonding orbitals of each energy level before occupying the antibonding.
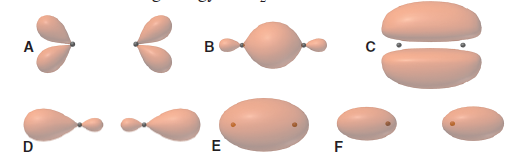
That is, an antibonding orbital is not an esoteric concept, but only a molecular orbital whose energy level is above the energy levels of the atomic orbitals from which it comes. You should consider the adjectives "bonding" and "antibonding" as descriptive of the energy levels of the electrons that will occupy those molecular orbitals. In contrast, the second MO obtained will be the antibonding orbital, in which the electrons will have an energy greater than the sum of the energies that they had separately.
a molecular orbital in which the electrons will have an energy less than the sum of the energies they had in the separated AOs. Therefore, the first will be a bonding orbital, i.e. One such combination stabilizes the electrons, while the other gives rise to a higher energy. That means that we are going to do a linear combination of two wave functions (the AOs) and that, therefore, we will get two new wave functions (the MOs). When the combination of two atomic orbitals is generated to obtain molecular orbitals, the most common procedure is to use the LCAO method.


 0 kommentar(er)
0 kommentar(er)
